Get Your Free Consultation
Bair Hugger Forced Air-Warming Blanket
Bair Hugger Forced Air-Warming Blanket: How It Works, How It Can Be Dangerous, and Lawsuits Against It
When a patient is under anesthesia, they need to have a regulated body temperature. If the patient gets too cold, there could be complications during the surgery. The Bair Hugger Forced Air-Warming (FAW) blanket was created as a solution to that problem, but it has caused complications of its own.
Get Your Free Consultation
Were You Harmed By Bair Hugger Forced Air-Warming Blanket?
Get Your Free Case Review
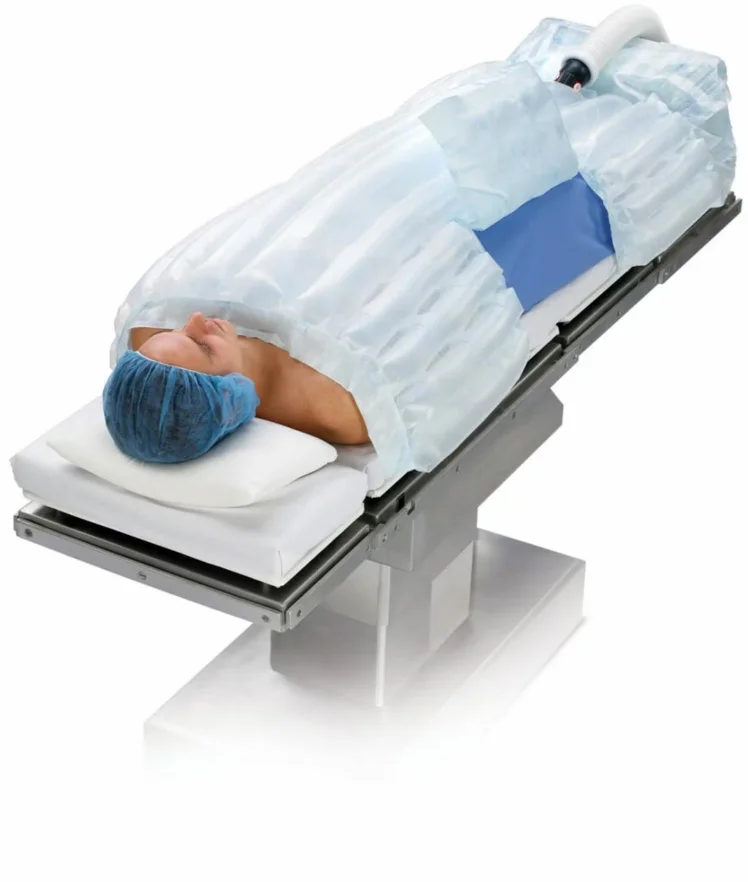
How Does a Bair Hugger Blanket Work?
During the procedure, the surgeon drapes a Bair Hugger blanket over the patient’s anesthetized area. The blanket has a system that circulates warm air through its components to control the patient’s body temperature. Keeping the patient warm is essential during surgery because the danger of hypothermia in the anesthetic area is raised. This process is especially important during knee or hip replacement surgeries.
The Bair Hugger blanket itself has two parts: the blanket and the hose attachment. The first is a disposable blanket that wraps around the patient. This covering should only be used once before it is discarded. The hose attachment connects to a warming unit, which heats the air before pumping it through the blanket to keep the patient warm. The small perforations in the blanket enable the warm air to pass safely onto the patient, and the cover traps the heat to keep the temperature regulated.
Who Manufactures Bair Huggers?
The FDA approved the first air-warming blanket manufactured by Augustine Medical, Inc., in 1987. Eventually, the company changed its name to Arizant, but in 2009, GM acquired Arizant as a subsidiary. However, 3M began to market and sell Bair Hugger blankets under their own name in 2010, making them the manufacturer and distributor of these medical devices.
How Is the Bair Hugger Warming Blanket Dangerous?
Without conducting any clinical trials, the FDA originally approved the Bair Hugger Forced Air Warming (FAW) system in 1987. According to an FDA precedent, if a piece of medical equipment is sufficiently comparable to previous devices that have already been approved, it can be cleared without a trial.
Unfortunately, the risks of this device were not documented before its FDA approval and subsequent release. Most notably, there is an increased risk of infection due to the device’s interference with the airflow present in operating rooms.
Because of the sterilized nature of an operating room, airflow is a critical part of maintaining filtration. By circulating air continuously, bacteria are prevented from settling in any of the patient’s exposed wounds. The Bair Hugger FAW blanket interrupts this critical airflow by producing air warmer than the rest of the room, thereby settling in the room and enabling bacteria to be carried to the operating table.
Even the most experienced Filevine users know first-hand how utterly confusing it can be to navigate its many features. This is especially true when you first set up your account. Filevine provides a very basic customizable setup, but this means that you will need to dedicate a significant amount of time to customize it.
Wired Help can not only help you begin the process to set up your account, but easily walk you through the many tools and features that will make your day-to-day work life easier.
After the initial set up, Wired Help can immediately boost productivity with complete development and customization of your Filevine. We will equip your team with expedient collaboration, centralize your case information, and integrate other apps and platforms. Eliminate the hassle and headaches. Spend your time doing what you are best at. Leave the tech to us!
3M has not made any public statements concerning the hazards associated with its products. However, a public study released in 2011 discussed the potential risks of using FAW systems in the sterile conditions of an operating room.
The original creator of the Bair Hugger also wrote a letter to company executives at Arizant after he departed, outlining his concerns for the product. Despite these warnings, 3M has declined to take steps to address the issues with the product.
Although the FDA has not issued a formal recall on Bair Hugger products, 3M did issue a voluntary recall in 2018, removing 165,000 products from use. 3M stated that a change in the Bair Hugger’s design caused some devices not to properly inflate. Patients developed hypothermia as a result of the device’s failure to regulate patients’ body temperatures adequately due to this product flaw. The voluntary recall only dealt with faulty equipment that could cause hypothermia and did not deal with complaints about infection.
What Lawsuits Have Been Filed Against the Bair Hugger?
Lawsuits as far back as 2013 have been filed against 3M for issues related to the Bair Hugger FAW Blanket. By 2015, one multidistrict case, including 14 plaintiffs against 3M had been created. Nearly 6,000 cases were filed with courts across the country in 2019. Initially, the US District Judge ruled that the plaintiffs in those cases did not have enough evidence to continue, but the ruling was reversed. By 2022, 3M had appealed the decision in front of the U.S. Supreme Court, but the court rejected their appeal.As of 2023, more than 6,000 lawsuits against 3M’s Bair Hugger FAW Blanket are being brought before state and federal courts.
What Compensation Can I Recover?
There are plenty of avenues for receiving compensation in a lawsuit against the Bair Hugger FAW Blanket, including:- Medical expenses, including prescriptions
- Rehabilitation expenses
- Lost wages/benefits
- Non-economic losses (pain, suffering, and other hardships)
- Funeral expenses and loss of consortium (in case of death)
You must speak with an experienced product liability attorney to ascertain what damages you may be entitled to. Make sure to pursue your case as soon as you can because there is a statute of limitations in effect on these cases. Most states have either a two or three-year statute, so make sure that you follow your state’s guidelines on filing.
How Can a Van Law Firm Help Me with My Case?
We understand that pursuing a legal case can be daunting, especially financially. Our firm believes that every client deserves justice, regardless of their financial situation. That is exactly why we take our cases on a contingency basis.
This means that you are not obligated to cover any upfront legal costs. Instead, we take our fees out of anything you may recover as a result of a successful settlement. This payment strategy enables you to file a lawsuit without worrying about how you’ll pay upfront. This financial independence makes it possible for us to pursue justice on your behalf.
You can be certain that you and your case will be taken care of and treated with respect when you contact Van Law Firm for legal assistance. Our award-winning team of attorneys can review your claim. Your consultation is always free. Call us today to begin the process of reviewing your case.
Free Phone Consultations, No Upfront Fees
Bair Hugger – Van Law Firm. All rights reserved.
